12.2.4.1 POJA-L2965+2963
Title: The organ of Corti in the inner ear
Description:
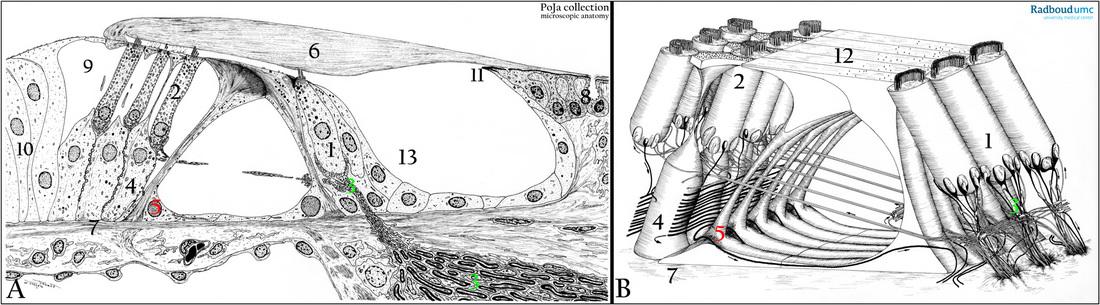
Theoretical explanation on the basis of the schemes. (A, B): Scheme of the organ of Corti and 3-D-scheme of the hair cells, human.
Both types of hair cells (Inner HCs and Outer HCs) in the ear are characteristically arranged in rows and are studded with nonmotile rigid stereocilia that are arranged in a characteristic V-pattern (an upwards slope of short to long cilia).
These receptor hair cells also possess a single kinocilium that will disappear in the adulthood with exception of those in the vestibular labyrinth (see further sacculus and utriculus).
All these sensory cells (=hair cells) in the organ of Corti are differentiated – so no regenerative capacity is possible - and fixed in their position by associated supporting phalangeal cells. All the stereocilia of the HCs emerge from a dense actin terminal web in the cell apex
(apical cuticular plate). Neighbouring stereocilia are connected by tip-links, a filamentous protein structure that interlinks the stereocilia and ion channels.
An inner hair cell (IHC) shows ca. 60 stereocilia in 2-3 rows linearly arranged on the apex.
In contrast an outer hair cell (OHC) exhibits ca 100-200 anchored 3 rows of stereocilia arranged in a W-shape of which the top is directed toward the stria vascularis.
(1) Inner hair cell (IHC), one single row of ca. 3500 IHCs.
(2) Outer hair cells (OHCs) in 3-4 parallel rows with a total of ca 12,000 OHCs.
(3) The hair cells are planted in a basket of nerve endings.
(4) Supporing cell (Deiters cell) with phalanx. The apical ends of the HCs are clasped by long filamentous processes of the supporting cells. These Deiters cells form at their top a kind of a roof through which the stereocilia of the hair cells extend and remain fixed in their position, the so-called reticular lamina or reticular plate (B, 12).
(5) The pillar cells are also a supportive cell type and create a tunnel (inner tunnel) through which nerve fibres run.
This inner tunnel (tunnel of Corti) is filled with endolymph.
(6) Tectorial membrane consisting of glycosaminoglycans, otogelin and the specific alpha- and beta tectorin proteins in gel form.
Otogelin is a specific protein synthesized by nonsensory cells ( 8) located underneath the acellular membranes such as tectorial membrane.
In maturing stages otogelin labels in thick and spaced radial fibre-like structures.
Note: when the alpha-tectorin encoding gene is mutated, deafness occurs.
(7) Basilar membrane.
(8) Supportive interdental cells form and renew as well as maintain the tectorial membrane.
(9) Outer tunnel between Hensen en OHCs.
(10) Hensen cells.
(11) At the vestibular lip of the spiral limbus the tectorial membrane extend to the OHCs.
(12) See in (B).
(13) The inner sulcus is lined by one layer of flat epithelial cells, that toward the IHCs become the so-called border cells.
Background: Afferent fibres (B, darker-coloured dendrites): about 95 % of the afferent dendrites (type I, myelinated) of the 8th nerve (cochlear part) originate from the base of the IHCs but only 5% of the afferent nerves (type II, unmyelinated) supply the OHCs.
The perikarya of the afferent fibres are localised in the spiral ganglion. From the large afferent endings on the IHCs the dendrites go straight to the habenula perforata. From the small afferent endings on the OHCs the dendrites firstly slope downwards, lay between the Deiter cells (i.e. the external spiral bundle). Near each outer pillar cell a dendrite turns off, and between the bases of these pillar cells subsequently traverses as a basilar fibre the inner tunnel (tunnel of Corti) toward the bases of the inner pillar cells and eventually goes through the habenula perforata.
Efferent fibers (B, light-coloured axons): the neurons from the superior olivary complex (brainstem) send their axons via the olivocochlear nerve into the cochlea. The axons traverse between the inner pillar cells through the inner tunnel of Corti and innervate the OHCs.
Most efferent axons synapse directly at the base of the OHCs. On the contrary, the IHCs show that their efferent axons only synapse on the afferent dendrites and not directly on their cell bodies. Afferent endings at the basal poles of IHCs and OHCs correspond with cytoplasmic synaptic ribbons (Ca2+ channels) respectively efferent endings with cytoplasmic postsynaptic cisternae.
All IHCs are localised in a high K+ ion concentrated endolymph giving rise to a voltage gradient between the ciliary tip to the base of the cell. Every IHC shows on its apex neighbouring cilia that progressively increase in height, they are all linked by tip-links (a thin stretchy filamentous protein) to each other.
The key molecules involved in the transduction activity of the hair bundle are protocadherin 15, cadherin 23 as well as myosin VI in the apical cuticular plate.
On the top of each cilium a mechanically gated ion channel is localised. Opening of the ion channel of a shorter cilium occurs when the neighbouring taller cilium bends away from the shorter one (positive displacement). The result is a quick K+ ion flow into each IHC when the ion channel opens resulting in a positive voltage of the IHC. Subsequently a neurotransmitter is released by the IHCs and sensory nerves at its basal poles are stimulated so the cochlear nerve generates action potentials. The stronger the decline of the voltage the lesser
the release of neurotransmitter and the greater the decrease in generated action potentials.
If the cilium bends toward its shorter neighbour (negative displacement), the ion channel closes resulting in a negative IHC voltage.
It appears that IHCs are the true sensory cells that transmit the impulses via the auditory nerve. They transform the cochlear fluid vibration (mechanical force) into action potentials in the nerve (electrical impulse) to be ‘interpreted’ in the brain.
OHCs do not react on physical sound vibration, in response to voltage changes of the cell membranes they elongate very quickly and actively (electromotile) and by doing so the basilar membrane motion is amplified (even up to 220,000 x per second).
It is assumed that the changes of vibration of the basilar membrane may improve and also tune the IHCs stimulation.
The function of the OHCs is qualitative amplification as they increase the selectivity and quantitative amplification and they also increase IHCs sensitivity to sound intensities (discrimination between various pitches of sound). See also: www.cochlea.eu
Keywords/Mesh: inner ear, organ of Corti, endolymph, hair cell, stereocilium, phalangeal cell, basilar membrane, efferent nerve,
afferent nerve, histology, electron microscopy, POJA collection
Title: The organ of Corti in the inner ear
Description:
Theoretical explanation on the basis of the schemes. (A, B): Scheme of the organ of Corti and 3-D-scheme of the hair cells, human.
Both types of hair cells (Inner HCs and Outer HCs) in the ear are characteristically arranged in rows and are studded with nonmotile rigid stereocilia that are arranged in a characteristic V-pattern (an upwards slope of short to long cilia).
These receptor hair cells also possess a single kinocilium that will disappear in the adulthood with exception of those in the vestibular labyrinth (see further sacculus and utriculus).
All these sensory cells (=hair cells) in the organ of Corti are differentiated – so no regenerative capacity is possible - and fixed in their position by associated supporting phalangeal cells. All the stereocilia of the HCs emerge from a dense actin terminal web in the cell apex
(apical cuticular plate). Neighbouring stereocilia are connected by tip-links, a filamentous protein structure that interlinks the stereocilia and ion channels.
An inner hair cell (IHC) shows ca. 60 stereocilia in 2-3 rows linearly arranged on the apex.
In contrast an outer hair cell (OHC) exhibits ca 100-200 anchored 3 rows of stereocilia arranged in a W-shape of which the top is directed toward the stria vascularis.
(1) Inner hair cell (IHC), one single row of ca. 3500 IHCs.
(2) Outer hair cells (OHCs) in 3-4 parallel rows with a total of ca 12,000 OHCs.
(3) The hair cells are planted in a basket of nerve endings.
(4) Supporing cell (Deiters cell) with phalanx. The apical ends of the HCs are clasped by long filamentous processes of the supporting cells. These Deiters cells form at their top a kind of a roof through which the stereocilia of the hair cells extend and remain fixed in their position, the so-called reticular lamina or reticular plate (B, 12).
(5) The pillar cells are also a supportive cell type and create a tunnel (inner tunnel) through which nerve fibres run.
This inner tunnel (tunnel of Corti) is filled with endolymph.
(6) Tectorial membrane consisting of glycosaminoglycans, otogelin and the specific alpha- and beta tectorin proteins in gel form.
Otogelin is a specific protein synthesized by nonsensory cells ( 8) located underneath the acellular membranes such as tectorial membrane.
In maturing stages otogelin labels in thick and spaced radial fibre-like structures.
Note: when the alpha-tectorin encoding gene is mutated, deafness occurs.
(7) Basilar membrane.
(8) Supportive interdental cells form and renew as well as maintain the tectorial membrane.
(9) Outer tunnel between Hensen en OHCs.
(10) Hensen cells.
(11) At the vestibular lip of the spiral limbus the tectorial membrane extend to the OHCs.
(12) See in (B).
(13) The inner sulcus is lined by one layer of flat epithelial cells, that toward the IHCs become the so-called border cells.
Background: Afferent fibres (B, darker-coloured dendrites): about 95 % of the afferent dendrites (type I, myelinated) of the 8th nerve (cochlear part) originate from the base of the IHCs but only 5% of the afferent nerves (type II, unmyelinated) supply the OHCs.
The perikarya of the afferent fibres are localised in the spiral ganglion. From the large afferent endings on the IHCs the dendrites go straight to the habenula perforata. From the small afferent endings on the OHCs the dendrites firstly slope downwards, lay between the Deiter cells (i.e. the external spiral bundle). Near each outer pillar cell a dendrite turns off, and between the bases of these pillar cells subsequently traverses as a basilar fibre the inner tunnel (tunnel of Corti) toward the bases of the inner pillar cells and eventually goes through the habenula perforata.
Efferent fibers (B, light-coloured axons): the neurons from the superior olivary complex (brainstem) send their axons via the olivocochlear nerve into the cochlea. The axons traverse between the inner pillar cells through the inner tunnel of Corti and innervate the OHCs.
Most efferent axons synapse directly at the base of the OHCs. On the contrary, the IHCs show that their efferent axons only synapse on the afferent dendrites and not directly on their cell bodies. Afferent endings at the basal poles of IHCs and OHCs correspond with cytoplasmic synaptic ribbons (Ca2+ channels) respectively efferent endings with cytoplasmic postsynaptic cisternae.
All IHCs are localised in a high K+ ion concentrated endolymph giving rise to a voltage gradient between the ciliary tip to the base of the cell. Every IHC shows on its apex neighbouring cilia that progressively increase in height, they are all linked by tip-links (a thin stretchy filamentous protein) to each other.
The key molecules involved in the transduction activity of the hair bundle are protocadherin 15, cadherin 23 as well as myosin VI in the apical cuticular plate.
On the top of each cilium a mechanically gated ion channel is localised. Opening of the ion channel of a shorter cilium occurs when the neighbouring taller cilium bends away from the shorter one (positive displacement). The result is a quick K+ ion flow into each IHC when the ion channel opens resulting in a positive voltage of the IHC. Subsequently a neurotransmitter is released by the IHCs and sensory nerves at its basal poles are stimulated so the cochlear nerve generates action potentials. The stronger the decline of the voltage the lesser
the release of neurotransmitter and the greater the decrease in generated action potentials.
If the cilium bends toward its shorter neighbour (negative displacement), the ion channel closes resulting in a negative IHC voltage.
It appears that IHCs are the true sensory cells that transmit the impulses via the auditory nerve. They transform the cochlear fluid vibration (mechanical force) into action potentials in the nerve (electrical impulse) to be ‘interpreted’ in the brain.
OHCs do not react on physical sound vibration, in response to voltage changes of the cell membranes they elongate very quickly and actively (electromotile) and by doing so the basilar membrane motion is amplified (even up to 220,000 x per second).
It is assumed that the changes of vibration of the basilar membrane may improve and also tune the IHCs stimulation.
The function of the OHCs is qualitative amplification as they increase the selectivity and quantitative amplification and they also increase IHCs sensitivity to sound intensities (discrimination between various pitches of sound). See also: www.cochlea.eu
Keywords/Mesh: inner ear, organ of Corti, endolymph, hair cell, stereocilium, phalangeal cell, basilar membrane, efferent nerve,
afferent nerve, histology, electron microscopy, POJA collection