7.4 POJA-L-1785+1862
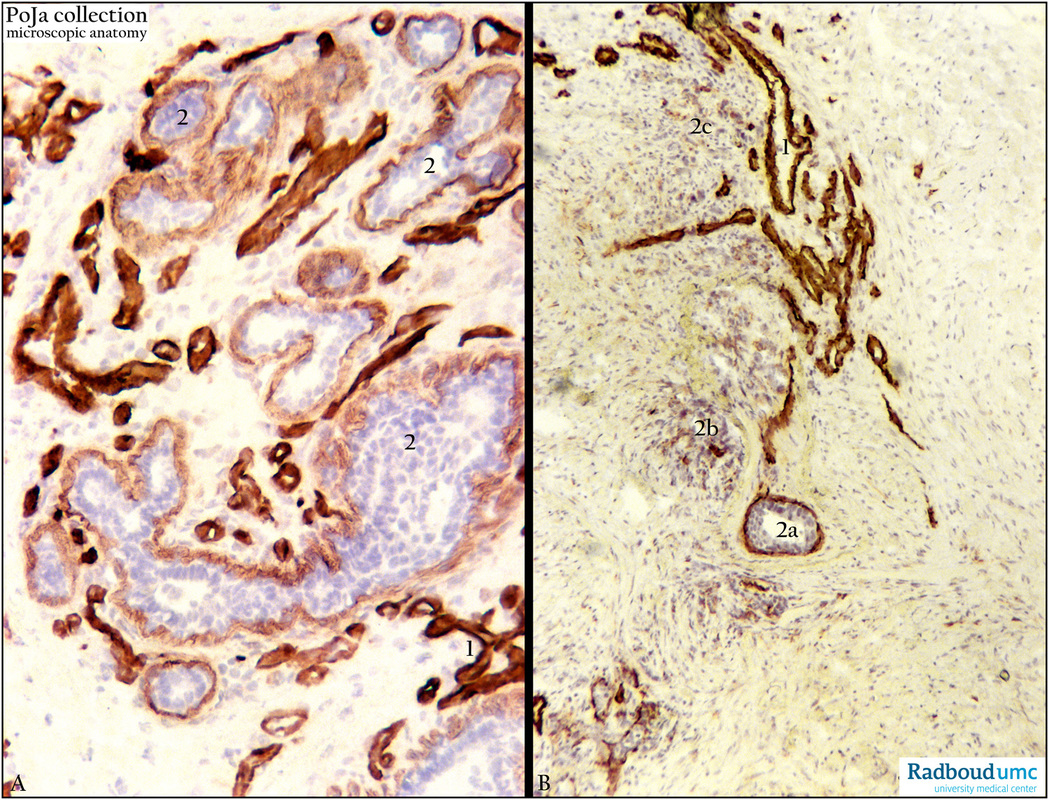
Title: Collagen and lamin staining of basement membrane of invasive breast carcinoma (human)
Description: Stain: (A) Antilaminin and (B) anticollagen-IV antibody immunoperoxidase staining with diaminobenzidin reaction (DAB) and hematoxylin counterstaining (frozen sections).
(A): Strong and regular anti-laminin staining of blood vessels (1). Infiltrating cords of tumor cells with luminization (2) exhibit finer outlined but still regular laminin reactivity.
(B): Linearly brown-stained basement membranes of blood vessels (1), tumor cell nest (2a) with luminization as well as small clusters shows irregular staining for collagen type IV indicating gradual loss of basement membrane collagen IV (probably indicating early signs of invasion). Dispersed infiltrating small groups of tumor cells (2c) show only patches of decreased intensity of collagen IV. (Stained sections supplied by R.H.W. Wetzels, PhD, former postdoc at the Department of Pathology and G.P. Vooijs MD PhD, former Head of the Department of Pathology, Radboud university medical center, Nijmegen, The Netherlands).
Background: Blood vessels in invasive breast carcinoma are known to be stained for type IV collagen, but the collagen IV-reactivity in tumor nodules appears significantly decreased (linear but discontinuous staining) or even absent. Irregularly stain deposits might indicate early disintegration of collagen type IV component in the basement membranes. Degradation by gelatinases belonging to the group of matrix metalloproteases (MMPs) is involved in the pathologic remodelling of the extracellular matrix (ECM) in tumor invasion and metastasis. Under mentioned reminder is a summary of the ultrastructure of the basement membrane and general functions of laminin/collagen type IV with few associated glycoproteins. In light microscopy a basement membrane separates the basal side of polarized epithelial or endothelial cells from the surrounding connective tissue (a.o. proper lamina). It encompasses two fused membranes i.e. the basal lamina (secreted by epithelial cells) and a close apposed reticular lamina (secreted by other cells). The latter is absent if a connective compartment lacks (e.g. kidney glomerulus, heart muscle). The basal lamina (lamina basalis) is a specialized form of extracellular matrix (ECM) and is often referred to as the type IV matrix. Ultrastructurally a basal lamina is composed of three sublayers: a clear or lucid layer (lamina lucida, lamina rara externa, ca. 60 nm thick) close to the epithelial cells, an intermediate electron-dense layer (lamina densa, average 30-100 nm thick) and a thin hardly electron-dense lamina rara interna (ca. 10 nm) closer to the connective tissue compartment. Within the electron-lucid layer laminins, integrins, entactins and dystroglycan are localized. The lamina densa contains a network of collagen IV coated with perlecan (a heparan sulfate proteoglycan). From the basal lamina anchoring fibrils type VII collagen extend from the rara interna with microfibrils into the apposed fibroreticular lamina (lamina fibroreticularis or sublamina densa zone, ca. 200-500 nm) containing type I/III collagen fibrils. The laminins form a family of large, non-collageneous glycoproteins that are secreted and incorporated into cell-associated ECM. They are localized in the lucid layer and anchor cell surfaces to basal laminae, form independent networks and associate with type IV collagen networks via perlecan and entactin. Integrins are transmembrane receptors that mediate attachment between cells and surrounding tissues and play a role in cell signalling. They regulate cell shape, motility and bind cell surface and ECM components such as laminin, collagen, fibronectin etc. Entactin belongs to the nidogen family, nidogen-1/-2 is a basal lamina glycoprotein that a.o. interacts with laminin, type IV collagen. Dystroglycan is a transmembrane protein, highly glycosylated and belongs to the dystrophin-associated glycoprotein complex. It is a receptor for multiple ECM molecules (a.o. laminin, perlecan) and plays a role in linking the ECM molecules to the actin cytoskeleton via the actin-binding protein dystrophin.
Keywords/Mesh: breast, mammary glands, breast neoplasms, breast carcinoma, ductal, lamin, collagen IV, metastasis, invasive, histology, POJA collection.
Title: Collagen and lamin staining of basement membrane of invasive breast carcinoma (human)
Description: Stain: (A) Antilaminin and (B) anticollagen-IV antibody immunoperoxidase staining with diaminobenzidin reaction (DAB) and hematoxylin counterstaining (frozen sections).
(A): Strong and regular anti-laminin staining of blood vessels (1). Infiltrating cords of tumor cells with luminization (2) exhibit finer outlined but still regular laminin reactivity.
(B): Linearly brown-stained basement membranes of blood vessels (1), tumor cell nest (2a) with luminization as well as small clusters shows irregular staining for collagen type IV indicating gradual loss of basement membrane collagen IV (probably indicating early signs of invasion). Dispersed infiltrating small groups of tumor cells (2c) show only patches of decreased intensity of collagen IV. (Stained sections supplied by R.H.W. Wetzels, PhD, former postdoc at the Department of Pathology and G.P. Vooijs MD PhD, former Head of the Department of Pathology, Radboud university medical center, Nijmegen, The Netherlands).
Background: Blood vessels in invasive breast carcinoma are known to be stained for type IV collagen, but the collagen IV-reactivity in tumor nodules appears significantly decreased (linear but discontinuous staining) or even absent. Irregularly stain deposits might indicate early disintegration of collagen type IV component in the basement membranes. Degradation by gelatinases belonging to the group of matrix metalloproteases (MMPs) is involved in the pathologic remodelling of the extracellular matrix (ECM) in tumor invasion and metastasis. Under mentioned reminder is a summary of the ultrastructure of the basement membrane and general functions of laminin/collagen type IV with few associated glycoproteins. In light microscopy a basement membrane separates the basal side of polarized epithelial or endothelial cells from the surrounding connective tissue (a.o. proper lamina). It encompasses two fused membranes i.e. the basal lamina (secreted by epithelial cells) and a close apposed reticular lamina (secreted by other cells). The latter is absent if a connective compartment lacks (e.g. kidney glomerulus, heart muscle). The basal lamina (lamina basalis) is a specialized form of extracellular matrix (ECM) and is often referred to as the type IV matrix. Ultrastructurally a basal lamina is composed of three sublayers: a clear or lucid layer (lamina lucida, lamina rara externa, ca. 60 nm thick) close to the epithelial cells, an intermediate electron-dense layer (lamina densa, average 30-100 nm thick) and a thin hardly electron-dense lamina rara interna (ca. 10 nm) closer to the connective tissue compartment. Within the electron-lucid layer laminins, integrins, entactins and dystroglycan are localized. The lamina densa contains a network of collagen IV coated with perlecan (a heparan sulfate proteoglycan). From the basal lamina anchoring fibrils type VII collagen extend from the rara interna with microfibrils into the apposed fibroreticular lamina (lamina fibroreticularis or sublamina densa zone, ca. 200-500 nm) containing type I/III collagen fibrils. The laminins form a family of large, non-collageneous glycoproteins that are secreted and incorporated into cell-associated ECM. They are localized in the lucid layer and anchor cell surfaces to basal laminae, form independent networks and associate with type IV collagen networks via perlecan and entactin. Integrins are transmembrane receptors that mediate attachment between cells and surrounding tissues and play a role in cell signalling. They regulate cell shape, motility and bind cell surface and ECM components such as laminin, collagen, fibronectin etc. Entactin belongs to the nidogen family, nidogen-1/-2 is a basal lamina glycoprotein that a.o. interacts with laminin, type IV collagen. Dystroglycan is a transmembrane protein, highly glycosylated and belongs to the dystrophin-associated glycoprotein complex. It is a receptor for multiple ECM molecules (a.o. laminin, perlecan) and plays a role in linking the ECM molecules to the actin cytoskeleton via the actin-binding protein dystrophin.
Keywords/Mesh: breast, mammary glands, breast neoplasms, breast carcinoma, ductal, lamin, collagen IV, metastasis, invasive, histology, POJA collection.