14.1 POJA-L6326+6327+6328+6329 Histochemistry-I of skeletal muscles (human)
 14.1 POJA-L6326+6327+6328+6329 Histochemistry-I of skeletal muscles
14.1 POJA-L6326+6327+6328+6329 Histochemistry-I of skeletal muscles
14.1 POJA-L6326+6327+6328+6329 Histochemistry-I of skeletal muscles (human)
(By courtesy of H. ter Laak PhD Section Neuropathology, retired staff member Department of Pathology, Radboud university medical center, Nijmegen, the Netherlands)
Title: Histochemistry-I of skeletal muscles (human)
Description:
Dystrophin, the protein product of the Duchenne muscular dystrophy (DMD) gene locus, is expressed on the muscle fibre surface. Dystrophin is a large, 427 kDa cytolinker protein that connects the interior of the cell to the extracellular matrix. While expressed in many tissues of the body, dystrophin has the critical role of stabilizing the muscle membrane (sarcolemma) during muscle contraction and its absence results in Duchenne muscular dystrophy (DMD).
See also
Merosin collectively is a term that refers to a group of laminins that shares the alpha2 subunit encoded by the LAMA2 gene. Laminins are a family of high molecular weight glycoproteins that function as extracellular matrix components of the structural basement membrane. They are heterotrimers composed of homologous alpha, beta, and gamma polypeptide chains. Individually, these chains have a distinct organisation of structural domains and can exhibit biological activities, including self-assembly and protein interaction. The alpha2 subunit is present in laminin 2 (merosin) and laminin 4 (s-merosin), two laminin types that play an integral role in skeletal muscle.
A mutation in the LAMA2 gene (on chromosome 6q) results in deficiency of laminin a2 in MDC1A (‘merosin deficient’ congenital muscular dystrophy 1A). In biopsies varying degrees of laminin expression are found, varying from total absence to traces or partial expression on the myofibres.
Spectrin colocalizes with dystrophin at the sarcolemma of the muscle cells. Skeletal muscle contains spectrin (or spectrin I) and fodrin (or spectrin II), members of the spectrin supergene family and they are associated with the sarcolemma structure. A good preservation of the sarcolemma is indicated by a uniform labelling on all fibres. On immature fibres it is reduced e.g., in neonates, juveniles. Necrotic fibres lack staining and regenerating fibres also show reduced activities.
Keywords/Mesh: locomotor system, skeletal muscle, striated muscle, sarcolemma, dystrophin, merosin, spectrin, histology, POJA-collection
Title: Histochemistry-I of skeletal muscles (human)
Description:
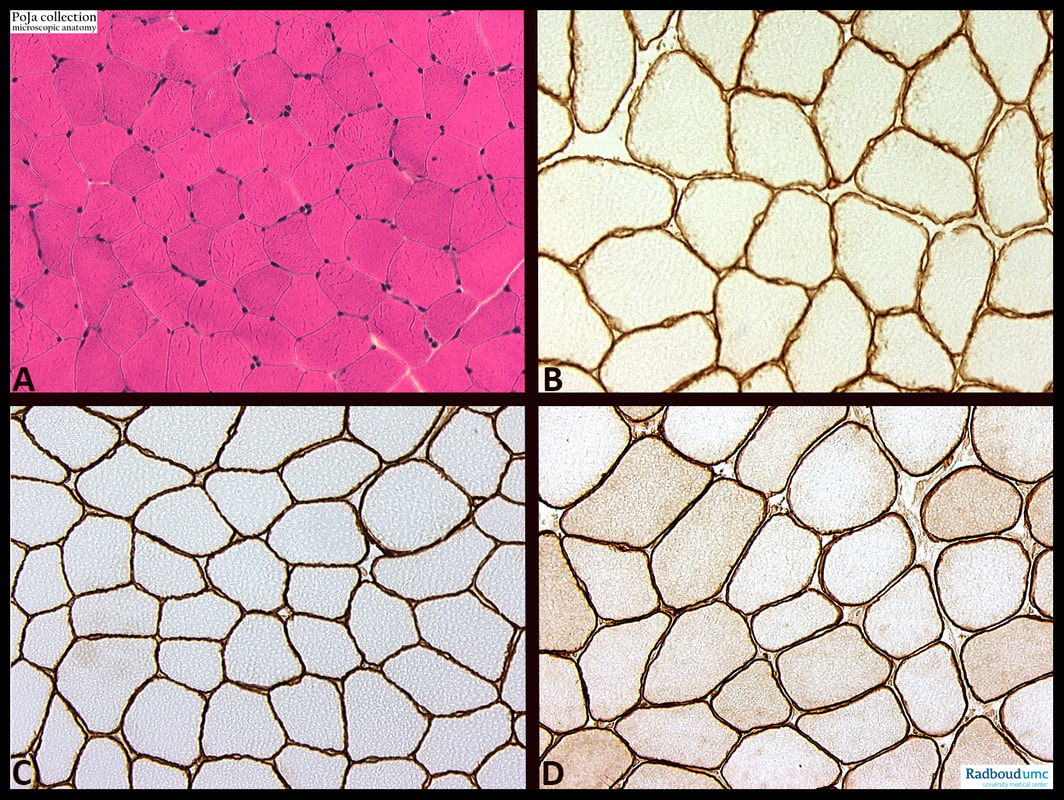
- (A): Haematoxylin-eosin staining.
- (B): Dystrophin immuno- peroxidase staining.
- (C): Merosin immunoperoxidase staining.
- (D): Spectrin immunoperoxidase staining.
Dystrophin, the protein product of the Duchenne muscular dystrophy (DMD) gene locus, is expressed on the muscle fibre surface. Dystrophin is a large, 427 kDa cytolinker protein that connects the interior of the cell to the extracellular matrix. While expressed in many tissues of the body, dystrophin has the critical role of stabilizing the muscle membrane (sarcolemma) during muscle contraction and its absence results in Duchenne muscular dystrophy (DMD).
See also
- 14.6.1 POJA-L6184+1685 Immunostaining for dystrophin in DMD
- 14.1 POJA-L6330+6331+6332+6333 Histochemistry-II of skeletal muscles
Merosin collectively is a term that refers to a group of laminins that shares the alpha2 subunit encoded by the LAMA2 gene. Laminins are a family of high molecular weight glycoproteins that function as extracellular matrix components of the structural basement membrane. They are heterotrimers composed of homologous alpha, beta, and gamma polypeptide chains. Individually, these chains have a distinct organisation of structural domains and can exhibit biological activities, including self-assembly and protein interaction. The alpha2 subunit is present in laminin 2 (merosin) and laminin 4 (s-merosin), two laminin types that play an integral role in skeletal muscle.
A mutation in the LAMA2 gene (on chromosome 6q) results in deficiency of laminin a2 in MDC1A (‘merosin deficient’ congenital muscular dystrophy 1A). In biopsies varying degrees of laminin expression are found, varying from total absence to traces or partial expression on the myofibres.
Spectrin colocalizes with dystrophin at the sarcolemma of the muscle cells. Skeletal muscle contains spectrin (or spectrin I) and fodrin (or spectrin II), members of the spectrin supergene family and they are associated with the sarcolemma structure. A good preservation of the sarcolemma is indicated by a uniform labelling on all fibres. On immature fibres it is reduced e.g., in neonates, juveniles. Necrotic fibres lack staining and regenerating fibres also show reduced activities.
Keywords/Mesh: locomotor system, skeletal muscle, striated muscle, sarcolemma, dystrophin, merosin, spectrin, histology, POJA-collection
