16.0.5 POJA-L7205 LOCOMOTOR SYSTEM: BONE
INTRODUCTION-5: LONG BONES: ENDOCHONDRAL OSSIFICATION
INTRODUCTION-5: LONG BONES: ENDOCHONDRAL OSSIFICATION
|
Development of long bones via the predecessor of hyaline cartilage.
The elongated cartilage model covered by a layer of connective tissue (perichondrium) shows two symmetrical ends (epiphyses) and in between the diaphysis. The primary ossification centre is located in the diaphysis where formations of the bone collar and the endochondral ossification take place. Bones grows in width (collar) by appositional bone deposition of the subperiosteal lining (former perichondrium) in the process of intramembranous ossification. Longitudinal growth in apical as well as distal directions occurs via the endochondral ossification. Mesenchymal osteoblast precursor cells express a master transcription factor Runx2 necessary for endochondral ossification. In the absence of Runx2 there will be no bone formation as well as no vascular invasion. Shortly before or after birth secondary ossification centres develop within the areas of the epiphyses. This process is similar to that in the diaphysis. The non-ossifying segments are formed by the joint surfaces and the epiphyseal plates. |
Eighth week human embryo shows in the centre of the diaphysis the start of a primary centre of ossification as chondrocytes enlarge and become vacuolated. Subsequently parallel to the long axis of the diaphysis arrangements of swollen chondrocytes aggregate followed by hypertrophy of all cells.
The cartilage matrix calcifies and is impregnated with calcium salts. Due to the calcification the supply of nutrients is impaired resulting in degeneration and disappearance of most chondrocytes. Out from the periosteum (former perichondrium) tissue sprouts consisting of a.o. capillaries, osteoprogenitor cells and osteoblasts erode and invade the calcified matrix. The osteoprogenitor cells develop into osteoblasts producing osteoid and using the calcified matrix as scaffold.
Subsequently the osteoid mineralises into primary bone and gradually an intricate system of interconnected trabeculae with a central core of calcified cartilage matrix is formed.
The primary bone formed on the surface of the calcified cartilaginous spicules will be broken down by infiltrating osteoclasts. In this process trabeculae and parts of primary spongy bone are removed resulting in a primitive bone marrow cavity.
Together with the invading osteogenic buds from the periosteum haemopoietic stem cells appear and give rise to the formation of a primary bone marrow as well the vascularisation of a primitive bone marrow cavity.
From the centre of the diaphysis cartilage matrix calcification and ossification now extends in two directions toward both the epiphyses. This process is present in the foetal bones as well as in the growth zones (epiphyseal plates) of children and adolescents. The epiphysial plates are composed of proliferating and pre-hypertrophic chondrocytes as well as the layer of hypertrophic chondrocytes.
The cartilage matrix calcifies and is impregnated with calcium salts. Due to the calcification the supply of nutrients is impaired resulting in degeneration and disappearance of most chondrocytes. Out from the periosteum (former perichondrium) tissue sprouts consisting of a.o. capillaries, osteoprogenitor cells and osteoblasts erode and invade the calcified matrix. The osteoprogenitor cells develop into osteoblasts producing osteoid and using the calcified matrix as scaffold.
Subsequently the osteoid mineralises into primary bone and gradually an intricate system of interconnected trabeculae with a central core of calcified cartilage matrix is formed.
The primary bone formed on the surface of the calcified cartilaginous spicules will be broken down by infiltrating osteoclasts. In this process trabeculae and parts of primary spongy bone are removed resulting in a primitive bone marrow cavity.
Together with the invading osteogenic buds from the periosteum haemopoietic stem cells appear and give rise to the formation of a primary bone marrow as well the vascularisation of a primitive bone marrow cavity.
From the centre of the diaphysis cartilage matrix calcification and ossification now extends in two directions toward both the epiphyses. This process is present in the foetal bones as well as in the growth zones (epiphyseal plates) of children and adolescents. The epiphysial plates are composed of proliferating and pre-hypertrophic chondrocytes as well as the layer of hypertrophic chondrocytes.
1. Zone of reserved cartilage (resting cartilage)
In the upper region of the plate small chondrocytes are randomly distributed.
2. Zone of proliferation (chondrocyte multiplication)
More distally in the plate toward the diaphysis chondrocytes arrange in rows parallel to the long axis of the diaphysis. Transversely dividing cells with mitotic activity are present as well as increased deposition of cartilage matrix between the new chondrocytes. The rate of progress of the proliferation zone is about the same as in the ossification zone.
3. Zone of hypertrophy
Chondrocytes enlarge by accumulation of glycogen and lipid droplets in the cytoplasm.
BMP2 produced by osteoblasts is after mediation by Runx2 also involved in pre-hypertrophic and hypertrophic chondrocytes. Blood vessels invade in between the hypertrophic chondrocytes at the periphery and these cells become apoptotic. Degeneration is indicated by enlarging of the nuclei, and becoming vacuolated or pyknotic lead to cell death creating lacunae distally in this zone. In growing bones few of this hypertrophic cartilage will survive as small islands of cartilage in the ossification centres.
RANKL, OPG and RANK are mostly expressed by hypertrophic chondrocytes, although there is also some contribution of pre-hypertrophic chondrocytes. It is recently suggested that hypertrophic chondrocytes are involved in the differentiation of osteoclast precursor at the growth plates. The expression of FRANKL appears regulated by BMP2 and vitamin D3, at least in vitro. In growth plates hypertrophic chondrocytes are located near newly-formed blood vessels where osteoclast precursors (OCP’s) derived from progenitors in liver and spleen circulate into developing bones. Hypertrophic chondrocytes a.o. Vascular Growth Factor (VEGF) that regulates vascular-in-growth and by FRANKL expression the efflux of OCP’s out of the blood vessels is also promoted due to the chemo-attractant effect of FRANKL on blood monocytes. In this way local release of FRANKL by chondrocytes and induction of its expression by BMP2 could mediate this OCP’s efflux.
4. Zone of calcified cartilage (ossification zone)
Developing osteoblasts deposit bone matrix on the surfaces of the cartilage islands resulting in bone struts (trabeculae). Flakes of cartilage matrix become impregnated by small calcifications. The transverse walls of the lacunae dissolve resulting in longitudinal spicules of calcified cartilage matrix. Osteoclasts appear not to be involved in the removal of cartilage as in RANK knockout mice cartilage also is resorbed. Presumably chondroblasts removed the cartilage but these cells are poorly defined and they might be from the monocyte lineage. Osteoblasts lay down osteoid and bone on the surface of longitudinal oriented calcified spicules and trabeculae. Gradually calcified cartilage transforms into a zone of cartilage removal as well as bone deposition.
The growth in both proliferation as well as ossification zone is equal. Sprouts with capillaries from the primary bone marrow grow into the lacunae supplying osteoprogenitor cells to these areas. Increased capillarisation contributes to resorption of the calcified cartilage matrix and subsequently to resorption of the partial bony spicules and trabeculae of the primary spongy bone. The OCP’s fuse with one another to form multinucleated osteoclasts resorbing most of the newly formed bone, subsequently only a few trabeculae left. In this way it comprises the spongy bone of the secondary spongiosa incorporating the metaphyseal bone between the epiphysis and the diaphysis of the bones. Due to osteoclastic resorption of the new bone more space is created for the proliferation and increasing number of hematopoietic cells derived from circulating precursors just like the OCP’s from the spleen and liver to contribute to the future bone marrow.
Longitudinal growth of the long bones: At time of birth to several years postnatally centres of secondary ossifications are formed in the epiphyses. The ossification process is similar to those in the diaphysis e.g., central localised chondrocytes of the epiphyses hypertrophy with calcification of the matrix. Out from the perichondrium buds of osteogenic tissue and capillaries enters the enlarged lacunae left behind by the degenerated chondrocytes. The ossification extends radially within the epiphysis with the formation of a small bone marrow cavity.
Between the epiphysis and the diaphysis, a layer of hyaline cartilage remains the so-called epiphysial plate. In human this plate allows further growth in length until 18-20 years.
In the upper region of the plate small chondrocytes are randomly distributed.
2. Zone of proliferation (chondrocyte multiplication)
More distally in the plate toward the diaphysis chondrocytes arrange in rows parallel to the long axis of the diaphysis. Transversely dividing cells with mitotic activity are present as well as increased deposition of cartilage matrix between the new chondrocytes. The rate of progress of the proliferation zone is about the same as in the ossification zone.
3. Zone of hypertrophy
Chondrocytes enlarge by accumulation of glycogen and lipid droplets in the cytoplasm.
BMP2 produced by osteoblasts is after mediation by Runx2 also involved in pre-hypertrophic and hypertrophic chondrocytes. Blood vessels invade in between the hypertrophic chondrocytes at the periphery and these cells become apoptotic. Degeneration is indicated by enlarging of the nuclei, and becoming vacuolated or pyknotic lead to cell death creating lacunae distally in this zone. In growing bones few of this hypertrophic cartilage will survive as small islands of cartilage in the ossification centres.
RANKL, OPG and RANK are mostly expressed by hypertrophic chondrocytes, although there is also some contribution of pre-hypertrophic chondrocytes. It is recently suggested that hypertrophic chondrocytes are involved in the differentiation of osteoclast precursor at the growth plates. The expression of FRANKL appears regulated by BMP2 and vitamin D3, at least in vitro. In growth plates hypertrophic chondrocytes are located near newly-formed blood vessels where osteoclast precursors (OCP’s) derived from progenitors in liver and spleen circulate into developing bones. Hypertrophic chondrocytes a.o. Vascular Growth Factor (VEGF) that regulates vascular-in-growth and by FRANKL expression the efflux of OCP’s out of the blood vessels is also promoted due to the chemo-attractant effect of FRANKL on blood monocytes. In this way local release of FRANKL by chondrocytes and induction of its expression by BMP2 could mediate this OCP’s efflux.
4. Zone of calcified cartilage (ossification zone)
Developing osteoblasts deposit bone matrix on the surfaces of the cartilage islands resulting in bone struts (trabeculae). Flakes of cartilage matrix become impregnated by small calcifications. The transverse walls of the lacunae dissolve resulting in longitudinal spicules of calcified cartilage matrix. Osteoclasts appear not to be involved in the removal of cartilage as in RANK knockout mice cartilage also is resorbed. Presumably chondroblasts removed the cartilage but these cells are poorly defined and they might be from the monocyte lineage. Osteoblasts lay down osteoid and bone on the surface of longitudinal oriented calcified spicules and trabeculae. Gradually calcified cartilage transforms into a zone of cartilage removal as well as bone deposition.
The growth in both proliferation as well as ossification zone is equal. Sprouts with capillaries from the primary bone marrow grow into the lacunae supplying osteoprogenitor cells to these areas. Increased capillarisation contributes to resorption of the calcified cartilage matrix and subsequently to resorption of the partial bony spicules and trabeculae of the primary spongy bone. The OCP’s fuse with one another to form multinucleated osteoclasts resorbing most of the newly formed bone, subsequently only a few trabeculae left. In this way it comprises the spongy bone of the secondary spongiosa incorporating the metaphyseal bone between the epiphysis and the diaphysis of the bones. Due to osteoclastic resorption of the new bone more space is created for the proliferation and increasing number of hematopoietic cells derived from circulating precursors just like the OCP’s from the spleen and liver to contribute to the future bone marrow.
Longitudinal growth of the long bones: At time of birth to several years postnatally centres of secondary ossifications are formed in the epiphyses. The ossification process is similar to those in the diaphysis e.g., central localised chondrocytes of the epiphyses hypertrophy with calcification of the matrix. Out from the perichondrium buds of osteogenic tissue and capillaries enters the enlarged lacunae left behind by the degenerated chondrocytes. The ossification extends radially within the epiphysis with the formation of a small bone marrow cavity.
Between the epiphysis and the diaphysis, a layer of hyaline cartilage remains the so-called epiphysial plate. In human this plate allows further growth in length until 18-20 years.
5. Growth and remodelling of bone occur throughout life
The principal events are:
(a): Early bone resorption.
(b): Growth in length and width;
(c): Conversion of spongy bone into compact bone;
(d): Remodelling of compact bone.
Ad 5a: Early bone resorption
During the growth process of the bone the resorption of the bone matrix occurs through the actions of the osteoclasts and the presence of blood capillaries. The main reason is to create space for the bone marrow cavity as well as to remove redundant trabeculae.
Ad 5b: Growth in length and width
Longitudinal growth occurs by continuous multiplication of chondrocytes within the epiphyseal plate. By appositional deposition of bone substance under the periosteum (former perichondrium) the bone enlarges in width. It is similar to the process of membranous ossification. The external circumferential lamellae provide an outer boundary for the cortical bone
Ad 5c: Conversion of spongy bone into compact bone
All primary bone is spongy and so all spongy bones of the diaphysis and the epiphyses are gradually converted into compact bone to increase the bone strength. Collagenous lamellae and mineralised bone matrix will be deposited on the surface of the cavities of the spongy bone. From one year postnatally in human vascular sprouts from the periosteum or bone marrow cavity grow into the compact bone.
Ad 5d: Remodelling of compact bone
In life-time the physical strain on cortical compact bones makes it essential to remodel existing osteons. The process is similar as described before. Vascular sprouts erode the compact bone and destroy partly Haversian systems. Subsequently new osteons are laid down as already described. The interstitial lamellae are remnants of partially destroyed osteons c.q. older eroded Haversian systems and separate osteons from one another.
The principal events are:
(a): Early bone resorption.
(b): Growth in length and width;
(c): Conversion of spongy bone into compact bone;
(d): Remodelling of compact bone.
Ad 5a: Early bone resorption
During the growth process of the bone the resorption of the bone matrix occurs through the actions of the osteoclasts and the presence of blood capillaries. The main reason is to create space for the bone marrow cavity as well as to remove redundant trabeculae.
Ad 5b: Growth in length and width
Longitudinal growth occurs by continuous multiplication of chondrocytes within the epiphyseal plate. By appositional deposition of bone substance under the periosteum (former perichondrium) the bone enlarges in width. It is similar to the process of membranous ossification. The external circumferential lamellae provide an outer boundary for the cortical bone
Ad 5c: Conversion of spongy bone into compact bone
All primary bone is spongy and so all spongy bones of the diaphysis and the epiphyses are gradually converted into compact bone to increase the bone strength. Collagenous lamellae and mineralised bone matrix will be deposited on the surface of the cavities of the spongy bone. From one year postnatally in human vascular sprouts from the periosteum or bone marrow cavity grow into the compact bone.
Ad 5d: Remodelling of compact bone
In life-time the physical strain on cortical compact bones makes it essential to remodel existing osteons. The process is similar as described before. Vascular sprouts erode the compact bone and destroy partly Haversian systems. Subsequently new osteons are laid down as already described. The interstitial lamellae are remnants of partially destroyed osteons c.q. older eroded Haversian systems and separate osteons from one another.
References
The above text is adapted from:
The above text is adapted from:
- Histology J.A.G. Rhodin 1974
- Histology A text and atlas M.H. Ross and W. Pawlina 2016 seventh edition Wolters Kluwer
- Anev Patel Types of Bones and from Bone Tissue AMBOSS 2021, Rosamond Nicolson and Jaspreet Johal, 2021 in teachmeanatomy.info/the-basics/ultrastructure/bone/)
- Ultrastructure of Bone - Components - TeachMeAnatomy
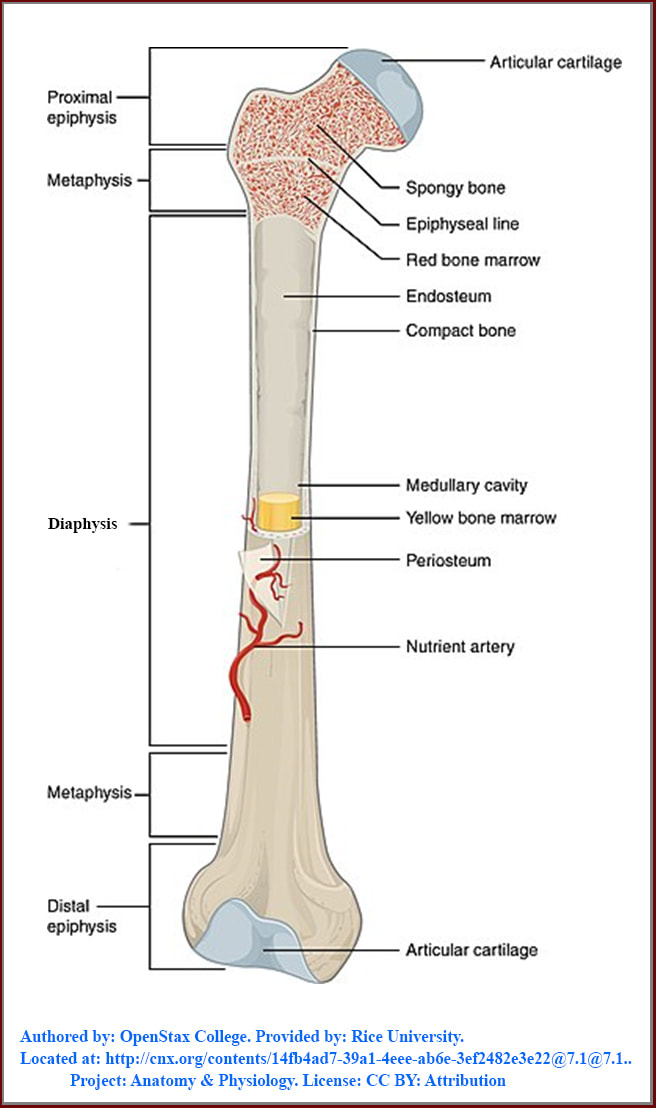
The bone figure:
CC licensed content, Shared previously
Authored by: OpenStax College. Provided by: Rice University. Located at: http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@7.1@7.1.. Project: Anatomy & Physiology. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/content/col11496/latest/.
CC licensed content, Shared previously
Authored by: OpenStax College. Provided by: Rice University. Located at: http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@7.1@7.1.. Project: Anatomy & Physiology. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/content/col11496/latest/.