14.1 POJA-L6297-B Anchoring of sarcolemma into the extracellular matrix (ECM)
14.1 POJA-L6297-B Anchoring of sarcolemma into the extracellular matrix (ECM)
(Skeletal muscle: A review of molecular structure and function, in health and disease Kavitha Mukund and Shankar Subramaniam, First published: 13 August 2019, https://doi.org/10.1002/wsbm.1462 © 2019 The Authors. WIREs Systems Biology and Medicine published by Wiley Periodicals, Inc. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.)
Title: Anchoring of sarcolemma into the extracellular matrix (ECM)
Description:
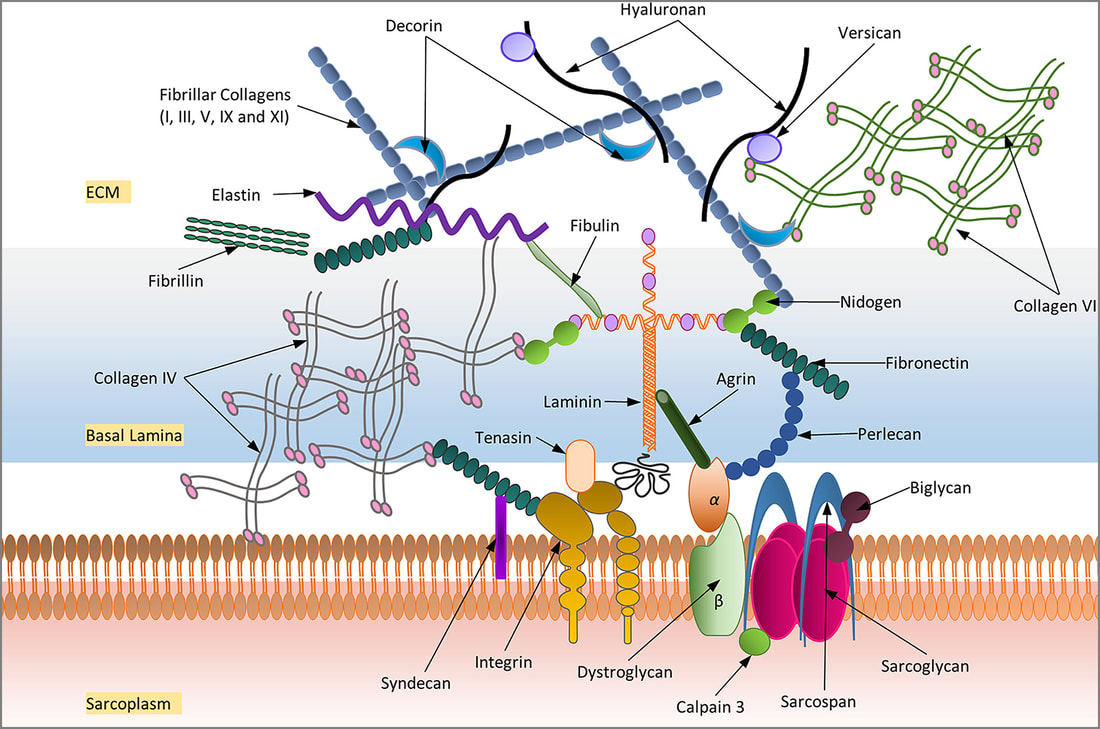
The extracellular matrix (ECM) of the skeletal muscle is organized in three discrete but interconnected layers that encapsulate the entire muscle: epimysium, perimysium and endomysium. The endomysium comprises a basal lamina adjacent to the sarcolemma and an outer reticular lamina and forms a delicate layer of ECM around each muscle fibre. The ECM constitutes three main classes of proteins:
The non-fibrillar collagen IV , however, forms the most important structural component of the basement membrane and integrates laminins, nidogens and others.
Fibronectin along with fibrillin-1 act as a bridge between integrins, collagen IV, PG’s and other focal adhesion molecules.
Elastin forms the elastic fibres.
The scheme shows a variety of many other proteins involved in the structure of the ECM (nidogens, periostin, SPP1, MMP’s) and PG’s such as hyaluronan, chondroitin sulphate, dermatan sulphate, versican, biglycan, decorin, lumican, fibromodulin) and HS PG’s (syndecan, perlecan, agrin).
The muscle cell or muscle fibre is anchored to the ECM with several molecules embedded in the sarcolemma, such as the sarcoglycans, sarcospans, dystroglycans, integrins, syndecans. These molecules are connected to the ECM via substances such as perlecan, agrin, laminins, fibronectins, tenascins and others.
Keywords/Mesh: locomotor system, skeletal muscle, striated muscle, sarcomere, cytoskeleton, costamere, sarcolemma, basal lamina, laminin, integrin, collagen fibre, fibrillar collagen I, non-fibrillar collagen IV, ECM, endomysium, scheme, electron microscopy, POJA collection
Title: Anchoring of sarcolemma into the extracellular matrix (ECM)
Description:
The extracellular matrix (ECM) of the skeletal muscle is organized in three discrete but interconnected layers that encapsulate the entire muscle: epimysium, perimysium and endomysium. The endomysium comprises a basal lamina adjacent to the sarcolemma and an outer reticular lamina and forms a delicate layer of ECM around each muscle fibre. The ECM constitutes three main classes of proteins:
- Collagens,
- Non-collagenous glycoproteins,
- Proteoglycans (PGs).
The non-fibrillar collagen IV , however, forms the most important structural component of the basement membrane and integrates laminins, nidogens and others.
Fibronectin along with fibrillin-1 act as a bridge between integrins, collagen IV, PG’s and other focal adhesion molecules.
Elastin forms the elastic fibres.
The scheme shows a variety of many other proteins involved in the structure of the ECM (nidogens, periostin, SPP1, MMP’s) and PG’s such as hyaluronan, chondroitin sulphate, dermatan sulphate, versican, biglycan, decorin, lumican, fibromodulin) and HS PG’s (syndecan, perlecan, agrin).
The muscle cell or muscle fibre is anchored to the ECM with several molecules embedded in the sarcolemma, such as the sarcoglycans, sarcospans, dystroglycans, integrins, syndecans. These molecules are connected to the ECM via substances such as perlecan, agrin, laminins, fibronectins, tenascins and others.
Keywords/Mesh: locomotor system, skeletal muscle, striated muscle, sarcomere, cytoskeleton, costamere, sarcolemma, basal lamina, laminin, integrin, collagen fibre, fibrillar collagen I, non-fibrillar collagen IV, ECM, endomysium, scheme, electron microscopy, POJA collection