16.1.3 POJA-L7102+7103+7106+7107 Endochondral ossification in foetus 5
16.1.3 POJA-L7102+7103+7106+7107 Endochondral ossification in foetus 5
Title: Endochondral ossification in foetus 5
Description:
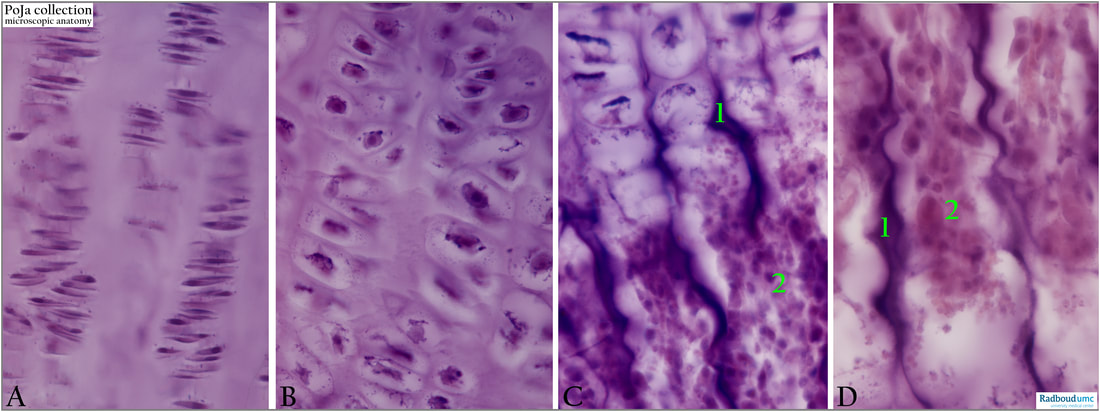
Foetal human bone, haematoxylin-eosin stain.
(A): Zone of proliferation of the chondrocytes. Cells remain arranged in columns during divisions.
(B): Zone of hypertrophy where cells enlarge.
(C): 0ssification zone, where chondrocytes are degraded and replaced by osteoblasts. The dark centers of the trabeculae (1) or spiculae are the remnants of the cartilage. (2): Bone marrow. The site of haematopoietic stem cells.
References
See also:
Keywords/Mesh: locomotor system, bone, endochondral ossification, foetus, cartilage, proliferation zone, hypertrophy zone, ossification zone, osteoblast, epiphysis, diaphysis, bone marrow, histology, POJA collection
Title: Endochondral ossification in foetus 5
Description:
Foetal human bone, haematoxylin-eosin stain.
(A): Zone of proliferation of the chondrocytes. Cells remain arranged in columns during divisions.
(B): Zone of hypertrophy where cells enlarge.
(C): 0ssification zone, where chondrocytes are degraded and replaced by osteoblasts. The dark centers of the trabeculae (1) or spiculae are the remnants of the cartilage. (2): Bone marrow. The site of haematopoietic stem cells.
- In the zone of proliferation (chondrocyte multiplication), i.e., more distally in the plate toward the diaphysis, chondrocytes arrange in rows parallel to the long axis of the diaphysis. Transversely dividing cells with mitotic activity are present as well as increased deposition of cartilage matrix between the new chondrocytes. The rate of progress of the proliferation zone is about the same as in the ossification zone.
- In the zone of hypertrophy chondrocytes enlarge by accumulation of glycogen and lipid droplets in the cytoplasm. Bone morphogenetic protein 2 or BMP-2 belongs to the TGF-β superfamily of proteins that induce osteogenesis by osteoblasts after mediation by Runx2 transcription factor that is involved in the regulation of cell cycle i.e., also involved in pre-hypertrophic and hypertrophic chondrocytes. Blood vessels invade in between the hypertrophic chondrocytes at the periphery and these cells become apoptotic. Degeneration is indicated by enlarging of the nuclei, and becoming vacuolated or pyknotic lead to cell death creating lacunae distally in this zone. In growing bones few of this hypertrophic cartilage will survive as small islands of cartilage (spicules) in the ossification centres. RANKL(-ligand), OPG and RANK (receptor activator of nuclear factor kB) are part of the signalling pathway that regulates osteoclast differentiation and activation, is involved in bone remodelling and repair, and is mostly expressed by hypertrophic chondrocytes, although there is also some contribution of pre-hypertrophic chondrocytes. It is recently suggested that hypertrophic chondrocytes are involved in the differentiation of osteoclast precursor at the growth plates. The expression of FRANKL appears regulated by BMP2 and vitamin D3, at least in vitro. In growth plates hypertrophic chondrocytes are located near newly-formed blood vessels where osteoclast precursors (OCP’s) derived from progenitors in liver and spleen circulate into developing bones. Vascular Growth Factor (VEGF) that regulates vascular-in-growth and by FRANKL expression the efflux of OCP’s out of the blood vessels is also promoted due to the chemo-attractant effect of FRANKL on blood monocytes. In this way local release of FRANKL by chondrocytes and induction of its expression by BMP2 could mediate this OCP’s efflux.
- In the zone of calcified cartilage (ossification zone) developing osteoblasts deposit bone matrix on the surfaces of the cartilage islands resulting in bone struts (trabeculae). Flakes of cartilage matrix become impregnated by small calcifications. The transverse walls of the lacunae dissolve resulting in longitudinal spicules of calcified cartilage matrix.
References
- Functions of RANKL/RANK/OPG in bone modelling and remodelling Brendan F. Boyce, Lianping Xing Arch Biochem Biophys. 2008, 5, 139–146 doi: 10.1016/j.abb.2008.03.018
See also:
- 16.1.3 POJA-L7095+7098+7096 Endochondral ossification in foetus 1
- 16.1.3 POJA-L7104+7105+7100 Endochondral ossification in foetus 2
- 16.1.3 POJA-L7134 Endochondral ossification in foetus 3a
- 16.1.3 POJA-L3813 Endochondral ossification in foetus 3b
- 16.1.3 POJA-L7109+7108+7101+7097 Endochondral ossification in foetus 4
- 16.1.3 POJA-L7147+7111+7110 Endochondral ossification in foetus 6
- 16.1.3 POJA-L7116+7115+7123 Endochondral ossification in foetus 7
- 16.1.3 POJA-L7145+7099 Endochondral ossification in foetus 8
- 16.0.5 POJA-L7205 Bone: introduction Bone formation-5 Long bones
Keywords/Mesh: locomotor system, bone, endochondral ossification, foetus, cartilage, proliferation zone, hypertrophy zone, ossification zone, osteoblast, epiphysis, diaphysis, bone marrow, histology, POJA collection